Publish date:
12 March 2021
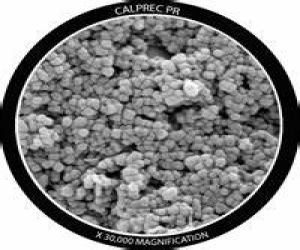
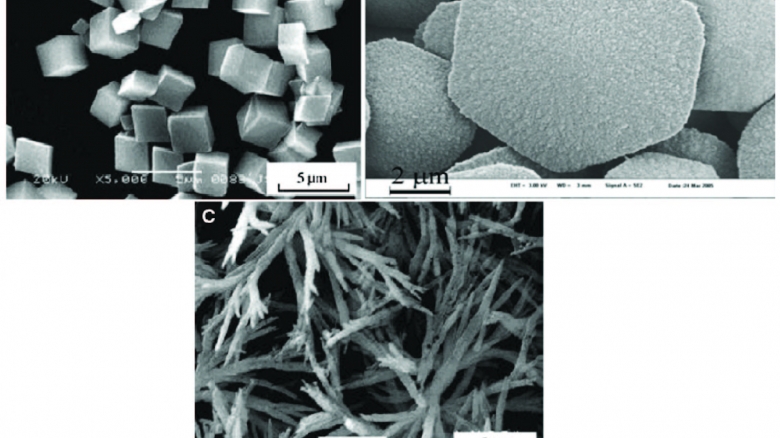
Calcium carbonate (CaCO3) is widely found in almost all living creatures, as well in some human tissues. Compared with other inorganic materials, over the years, CaCO3 has shown auspicious potential for the development of smart carriers for various anti-malignant neoplastic drugs. It is biodegradable, biocompatible and also a good pH-sensitive material. These properties make CaCO3 suitable for controlled degradability both in vitro and in vivo. Precipitated calcium carbonate can be handily and flawlessly produced in a precipitation reaction by reacting aqueous calcium hydroxide, Ca(OH)2 known as milk of lime (MOL) with carbon dioxide (CO2) (‘‘carbonation’’). Synthesis of PCC under certain reaction parameters, such as reaction temperature of 7–18 C, may yield ‘‘basic calcium carbonate’’ that, if desired, can be used as a forerunner for further conversion to other forms of calcium carbonate, such as aragonite or calcite, by increased carbonation. In most cases, basic calcium carbonate is a desirable form of the material because it has a ‘‘flaky’’ structure that is exceptionally good at imparting desirable functional properties such as whiteness, opacity and high gloss when prepared as part of filler in paint or paper. Generally, it is desired to produce precipitated calcium carbonate in specific forms and particle sizes such as the nanoparticle size calcite form. Calcite mineral exists in a trigonal crystalline form with crystal habits such as rhombohedral, hexagonal prism, scalenohedral, cubic and prismatic. These specific morphologies are necessary because the coating properties, such as light dispersion, of a calcium carbonate material are highly correlated to its morphology, structure and particle size. Precipitated calcium carbonate produced with a Prismatic and rhombohedral shape has maximum light dispersion at 0.4–0.5 lm sized particles. On the other hand, scalenohedral-shaped precipitated calcium carbonate has a maximum light dispersion of 0.9–1.5 lm particles. PCC of nanometer sized with rhombohedral morphology is highly effective for use as a coating on paper production. In food and foodstuffs industries, calcium carbonate is utilized not only because it provides the body system with an important nutrient (calcium), but also useful as a conditioner in the prevention of caking in food powders. Apart from food products, precipitated calcium carbonates are also used to a great degree in dentifrices, particularly toothpaste, where they serve as both abrasives and fillers. PCCs are much less expensive when compared to other dentifrice abrasives such as silica and di-calcium phosphate. PCCs have the same chemical formula with its precursor material such as limestone, chalk and marble; CaCO3 . PCCs have a lot of advantages over natural and ground calcium carbonate with their unique properties of smaller particle size, high purity, narrow particle size distribution and regular crystal shape. Unlike ground calcium carbonate, PCCs can be produced in different crystal shapes and in ultrafine particle sizes. PCC is increasingly used in industries such as paper, rubber, paint, textile, plastic, sealant, cosmetic, toothpaste and food mainly as a filler product. There is a continuing need for the production of precipitated calcium carbonate that will bestow maximum lifespan and optical performance properties to paper, paint, textile, etc., when included in their coating composition. The desired precipitated calcium carbonate materials should preferably be in a definite crystal form that is most likely to enhance such needed performance, and have other important properties such as particle size and particle size distribution that further enhance the performance in terms of brightness and durability of the end product. ISO brightness of 95% is required for a precipitated calcium carbonate coating pigment; this can only be achieved using very pure carbonate rocks as raw material. The world demand for precipitated calcium carbonate has been on the increase.
Precipitated calcium carbonate applications
Precipitated calcium carbonate is widely used as fillers, and finds uses in many products from asphalt to paper. The higher value, nano-grade PCC (top cut-off size 100 nm) is used as an efficient filler in the paper, pharmaceutical, paint, plastic, caulk and sealants industries.